Abstract
Background Necrotizing soft tissue infection (NSTI) is a life-threatening infection associated with high morbidity and mortality. Treatment consists of surgery and antibiotics. Many studies have addressed NSTI and its subtypes, but few have reviewed the clinical, radiological, and pathological differences between the polymicrobial and monomicrobial diseases. The objective of our study was to evaluate the clinical, radiological, and pathological features of patients with polymicrobial (NSTI I) and monomicrobial (NSTI II) infections and their association with outcome.
Methods The cohort consisted of patients hospitalized with NSTI at a tertiary medical center in 2002–2019. The medical charts were reviewed for clinical, radiological, and pathological features. Findings were compared between patients in whom blood/tissue bacterial cultures yielded one or more than one pathological isolate. The primary clinical outcome measure of the study was all-cause mortality at 90 days. Secondary outcomes were duration of hospitalization, intensive care unit (ICU) admission, score on the LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis), and need for vasopressor treatment.
Results A total of 81 patients met the inclusion criteria: 54 (66.6%) with monomicrobial NSTI and 27 (33.3%) with polymicrobial NSTI. There were no significant between-group differences in in-hospital and 90-day mortality. On multivariate analysis, the monomicrobial disease group had a significantly higher 90-day mortality rate in addition to higher rates of in-hospital mortality, ICU admission, and vasopressor use than the polymicrobial disease group.
Conclusion Our study is the first to compare the clinical, radiological, and pathological differences between the two most common types of NSTI. The results demonstrate better prognosis for polymicrobial NSTI, with minimal ICU stay, lower mortality, and lower use of vasopressors.
Level of evidence Prognostic and epidemiological, level III.
Introduction
Necrotizing soft tissue infection (NSTI) is an infrequent fatal soft tissue infection first described by Jones in 1871.1 The annual incidence in the USA is 0.04 per 1000 person-years with variations among different populations.2 3 Onset is sudden, followed by rapid spread, with mortality ranging from 30% to 70%.4–7 Therefore, early diagnosis is crucial.
NSTI is divided into several subtypes based on the microbiological profile.
Type I NSTI is a polymicrobial infection, involving aerobic and anaerobic organisms. While some studies have found it to be the most prevalent type, accounting for almost 80% of all NSTIs,8 9 other studies found its prevalence to be around 30% to 50%.5–7 Predisposing factors include diabetic ulcers, hemorrhoids, and rectal fissures. Gas in the tissue is often correlated with type 1 NSTI.10
Type II NSTI is a monomicrobial infection; the most prevalent pathogen is group A Streptococcus,3 11 followed by Staphylococcus aureus.10 11 Type II NSTI accounts for 10% to 40% of all NSTIs.8 9 It may occur in all age groups and in individuals with no comorbidities.12
Type III and IV NSTIs are rather scarce, and their definition is still debated. Type III is characterized by some authors as a clostridial infection13 whereas others characterize it as a monomicrobial infection caused primarily by Vibrio spp.8 14 Type IV is caused by a fungal infection after a traumatic wound or burns; Aspergillus and Zygomycetes are the most frequent causative organisms.14
Monomicrobial NSTI caused by Gram-negative bacteria has been reported more frequently in recent years,15–17 with some studies suggesting that it currently accounts for about 50% of all cases of monomicrobial NSTI. The predominant causative organisms are Klebsiella pneumoniae18 and Escherichia coli.16 17 19 The reported 30-day mortality rate for Gram-negative monomicrobial NSTI is 42.1% compared with 30.8% for Gram-positive NSTI.17
The diagnosis of NSTI depends on clinical symptoms, imaging findings, and exploratory surgery. Despite the many studies of NSTI, however, data comparing these features among the different disease types are sparse.
The aim of our study is to compare all-cause mortality at 90 days between the groups and to research, via a relatively large cohort, the clinical, radiological, and pathological characteristics of type I and II NSTIs.
Methods
The healthcare database of a tertiary medical center was searched for all patients hospitalized with NSTI (identified by the relevant International Classification of Diseases-9th Revision (ICD-9) codes including m72.6)20 between 2002 and 2019. The diagnosis was based on evidence of necrotic fascia during surgery or pathological features consistent with the diagnosis (extensive tissue destruction, blood vessel thrombosis, abundant bacteria spreading along fascial planes, and infiltration of acute inflammatory cells)21 with histopathological verification. All patients had CT scans as part of evaluation and all patients were taken to the operating room. Blood and involved soft tissue samples were obtained intraoperatively or by skin biopsy prior to antibiotic treatment and processed for aerobic and anaerobic bacterial cultures at the hospital’s microbiology laboratory. Bacterial identification and susceptibility testing were done by routine methods using manual biochemical methods followed by VITEK-II (Biomerieux, Marcy l’Etoile, France) in inconclusive cases until 2012, and thereafter, with verification in all cases by MALDI-TOF (Matrix-assisted laser desorption-ionization time of flight mass spectrometry, Shimadzu, Kyoto, Japan) or VITEK-II. Susceptibility tests were performed using disk diffusion and E-test methods and interpreted according to Clinical and Laboratory Standards Institute criteria.
For the present study, the cohort was divided into two groups according to the NSTI classification: polymicrobial infection (type I NSTI, Pm group) and monomicrobial infection (type II NSTI, Mm group). Although type III NSTI is also considered monomicrobial, we found that all causative monomicrobial organisms that were identified were characteristic of type II.
The medical files of each patient were reviewed for clinical and laboratory data as follows: demographics, vital signs, white cell count, platelet count, and levels of C-reactive protein, creatinine, serum glucose, lactate, creatine kinase, albumin, and sodium; comorbidities (identified by ICD-9 codes); findings on clinical examination and imaging; number of operations, duration of hospitalization, intensive care unit (ICU) admission, amputation, use of vasopressors, and date of death if applicable. As part of the clinical examination, patients were evaluated for comorbidities using the Charlson Comorbidity Index22 and severity of sepsis using the quick sequential organ failure assessment (qSOFA).23 24 Positive immunosuppression status was checked for all patients and was defined as use of more than 20 mg prednisone and/or cytotoxic drug/chemotherapy. The Laboratory Risk Indicator for Necrotizing soft tissue infection (LRINEC) was applied to distinguish severe cellulitis or abscess from necrotizing fasciitis.25 The LRINEC score is based on levels of C-reactive protein, white cell count, hemoglobin, sodium, creatinine and glucose; a score >6 or higher is considered diagnostic of NSTI.
Outcomes
The primary outcome measure of the study was all-cause mortality at 90 days. Secondary outcomes were length of hospitalization, ICU admission, LRINEC score, need for vasopressor, and amputation at 90 days.
Statistics
Data are expressed as mean and SD, median and IQR, or number and percentage. T-test, χ2 test, and non-parametric tests were used to compare clinical characteristics between the Pm and Mm groups. To estimate the association of the study outcomes with the bacterial type of growth, we have conducted a forward stepwise logistic regression of the dependent variable. Each set of covariates (demographic, medical history, laboratory, etc) was entered as a separate block into the model. The final model was selected on the basis of goodness of fit using the C-statistic and a plausible clinical explanation. The final models were adjusted for patient age and sex and Pm versus Mm. Cox regression with the same stepwise method was used to analyze 90-day mortality. Data were generated with SPSS V.25.0. A p value <0.05 was considered significant.
Results
Eighty-one patients with NSTI were included in the study, 54 (66.6%) with monomicrobial disease (Mm group) and 27 (33.3%) with polymicrobial disease (Pm group). In all cases, cultures of either blood or tissue or both grew bacteria. Table 1 depicts all pathogens isolated. In the Mm group, the majority of isolates were Gram-positive bacteria (31/54, 57.4%), mostly Streptococcus pyogenes (18/54, 33.3%). E. coli was the most common Gram-negative isolate (7/22, 13%). In the Pm group, Gram-negative bacteria predominated (38/54, 70%), with E. coli being the most common isolate (17/54, 31.5%). In some patients, cultures grew several types of bacteria such that the total number of isolates was larger than the number of patients (online supplemental file 1).
Supplementary data
General characteristics of patients with polymicrobial or monomicrobial NSTI
Table 1 shows the epidemiological characteristics of the cohort. A significantly higher proportion of the Pm group had a body mass index of >25 (p<0.001). There was no statistically significant difference between the Pm and Mm groups in mean qSOFA score (1.56±0.9 vs. 1.67±0.9, p=0.64, respectively), indicating similar sepsis severity. A significantly higher percentage of patients in the Mm group had undergone surgery in the last 30 days (47.4% vs. 16.7%, p=0.02).
Clinical findings such as redness and swelling were significantly more common in the Mm group (p=0.04 and p=0.01, respectively), whereas confusion was more common in the Pm group (p=0.04) (table 2).
Clinical characteristics of patients with polymicrobial or monomicrobial NSTI
Mean LRINEC scores were similar. More patients in the Pm group reported pain, but the difference was not statistically significant. There was no significant between-group difference in laboratory values except a higher platelet count in the Pm group. Creatine kinase levels were within normal range in both groups (online supplemental file 2).
Analysis of the radiographic features revealed that the Pm group had significantly more findings than the Mm group of free air (p=0.02) and fat infiltrates (p=0.002). On tissue biopsy studies, the Pm group had significantly higher rates of extensive tissue destruction and inflammatory cell infiltration (p=0.003 and p=0.01, respectively) (table 3).
Radiological characteristics of patients with polymicrobial or monomicrobial NSTI patient’s cohort
The clinical outcome results are shown in table 4. There was no significant difference between the groups in 90-day mortality. However, the Pm group was hospitalized for a significantly longer period than the Mm group and had undergone significantly more surgical interventions by 90 days (p=0.06).
Outcomes of patients with polymicrobial or monomicrobial NSTI
On multivariate analysis adjusted for patient age and sex, the OR of 90-day mortality was higher in the Mm than the Pm group, as were the ORs of hospital mortality, ICU admission, and vasopressor use (table 5).
Multivariate logistic regression of clinical outcomes by bacterial type*
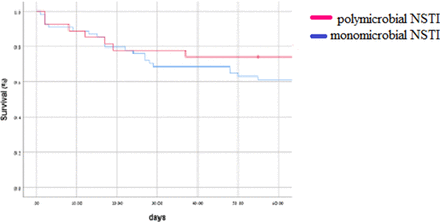
Figure 1 shows in-hospital mortality stratified by disease type. At the 90-day point, more patients in the Pm group had survived longer compared with patients in the Mm group (p=0.25), but the difference did not reach statistical significance.
Kaplan-Meier of overall mortality stratified by microbial type (p=0.23).
Discussion
This is to our knowledge the first study to evaluate clinical, laboratory, pathological, and outcome differences between type I and type II NSTIs. This information has implications for improving diagnosis and initiating earlier treatment, thereby decreasing the risks of morbidity and mortality.
Analysis of the clinical features of the patients revealed that overweight patients were more likely to have polymicrobial than monomicrobial disease, in line with previous studies.26 Redness and swelling at the site of infection was significantly more characteristic of monomicrobial disease whereas non-specific findings were more characteristic of polymicrobial disease. Accordingly, the current literature shows that group A Streptococcus pathogens, found more often in the Mm group, are often located in the extremities and in more exposed areas, whereas polymicrobial NSTI is more complex and causes deeper infections involving the perineum and sacrum where redness and swelling cannot be seen.26–28 Pain was common in both groups. It is not unusual that pain is not proportional to findings on physical examination.29
Several radiological findings overlapped between the groups. Non-specific findings included fluid collection, edema, and liquefaction. These agree with the known radiological pattern for NSTI. Studies have described fluid collections along the deep fascial sheaths, non-enhancement of the muscular fascia and extension of edema into the intramuscular septa, vascular thrombosis, and subcutaneous gas, in addition to low attenuation areas in the deeper fascial planes suggestive of fat infiltrate and liquefaction necrosis, with fascial involvement and lack of fascial enhancement being the most indicative signs of NSTI.30 In our study, the CT findings were consistent with the literature30 31: cases of free air in the tissue were more common in the Pm group, reflecting the anaerobic component underlying this finding.32
The post-treatment findings were better in the Pm group, namely lower rates of ICU admission, use of vasopressors, and in-hospital mortality. Several studies have reported an association of monomicrobial Gram-negative NSTI33 34 and group B Streptococcus NSTI25 with higher mortality rates. Our Mm group included six patients with NSTI caused by group B Streptococcus. Nevertheless, historically, group A Streptococcus accounts for most cases of monomicrobial NSTI. It is possible that the higher mortality rate in the Mm group, although not statistically significant, could be attributable to an epidemiological shift of invasive group A Streptococcus strains as was described in studies from Sweden.26 35 36
The higher mortality in the Mm group might also be related to the higher rate of surgical interventions performed on the Pm group. This finding has not been described previously in the literature.
Limitation
Our study has several limitations. First, NSTI is a rare disease; hence, the number of cases in this cohort is small; however, most of the published studies dealing with NSTI are of the same size. Second, the retrospective nature of this study limits us to the available medical records, hence, decision-making data on the reasons why some patients underwent more operations than others did, and on the nature of the medical treatments including ventilation support, hemodynamic support and antibiotic treatment, are not noted. Our study is based on the medical charts, filled by medical staff, it is possible that there were some mistakes; however, it is unlikely that such errors would have occurred more often in a particular group.
Conclusion
In conclusion, our study is the first to compare the two most prevalent types of NSTI in terms of clinical and radiological characteristics and outcome. The results demonstrate better prognosis for polymicrobial than monomicrobial NSTI, with minimal ICU stay, lower mortality, and lower use of vasopressors. Those results can help the physicians to detect earlier NSTI either by the unique radiological features accompanying type I NSTI and/or solely by raising the awareness to the microbiology and laboratory features in hope for better prognosis. Further investigations are warranted to assess the true diagnostic and therapeutic impacts of our findings.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
Helsinki Ethics Committee of Rabin Medical Center (approval number: RMC-17-194).
Footnotes
EN and SS contributed equally.
Contributors EN, HD-B, and SS recorded the patient characteristics. SS, JB, and DY wrote the article. IS and NG performed the statistical analysis. MD drafted and critically revised the article.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; internally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.