Abstract
Background ‘Community consultation’ (CC) is a key step when conducting Exception From Informed Consent research. Social-media-based CC has been shown to reach more people than traditional methods, but it is unclear whether those reached are representative of the community as a whole.
Methods This is a retrospective analysis of the CC performed in preparation for the PHOXSTAT trial. Social media advertisement campaigns were conducted in the catchment areas of the three participating trauma centers and evaluated by examining Facebook user statistics. We compared these data to georeferenced population data obtained from the U.S. Census Bureau. We examined variations in the proportion of each age group reached, by gender.
Results Our social media advertisements reached a total of 332 081 individuals in Los Angeles, Birmingham, and Nashville. Although there were differences in the proportion of individuals reached within each age group and gender groups, compared with the population in each area, these were small (within 5%). In Birmingham, participants 55 to 64 years old, 25 to 34 years old, and females 18 to 24 years old were slightly over-represented (a larger proportion of individuals in this age group were reached by the social media campaign, compared with the population resident in this area). In contrast, in Nashville, female participants 45 to 64 years old, and males 25 to 64 years old were over-represented. In Los Angeles, females 45 to 64 years old, and males 25 to 64 years and over were over-represented.
Discussion In conclusion, this study demonstrates that social media CC campaigns can be used to reach a sample of the community broadly representative of the population as a whole, in terms of age and gender. This finding is helpful to IRBs and investigators, as it lends further support to the use of social media to conduct CC. Further work is needed to analyze how representative community samples are in terms of other characteristics, such as race, ethnicity, and socioeconomic status.
Level III evidence Economic & Value-based Evaluations.
Background
Randomized clinical trials are the most rigorous way to evaluate the efficacy of an intervention and are essential to advancing medical care. Clinical trials must be conducted in accordance with accepted ethical principles, and respect for patients’ autonomy is a central tenet of the Declaration of Helsinki.1 Usually, participation is voluntary, and consent must be informed by a discussion, and understanding, of the benefits and risks of taking part. This is problematic when conducting research in emergency care.
In 1996, recognizing the importance of such research, the Food and Drug Administration (FDA) and the Department of Health and Human Services (DHHS) announced a waiver of the 21 CFR 50.24 and 45 CFR Part 46 requirements for obtaining and documenting informed consent. This only applies to a strictly limited class of research, involving research activities that may be performed in human subjects who are in need of emergency therapy and for whom, because of the subjects’ medical condition and the unavailability of legally authorized representatives of the subjects, no legally effective informed consent can be obtained.2
The DHSS and FDA have issued guidelines regarding the execution of such studies.3 One of the key steps is ‘community consultation’ (CC), a process that involves consultation between the investigators, the Institutional Review Board (IRB), and community members in the area where the research will take place. CC should not be mistaken for community consent. Consulting with a community includes eliciting feedback, criticism, and suggestions. It does not include asking for approval or permission.4
The regulations do not specify how the CC should be conducted, but traditional approaches involve attending community forums, arranging public meetings, newspaper and television advertisements, and random digit dialing telephone surveys. These methods are time-consuming, costly, and only reach small and often (self-) selected segments of a community.5 Little data are available assessing whether the traditional methods reach the appropriate members of the community.
A more contemporary way of conducting CC is to use social media.5–7 More than half (190 million) of US residents have a Facebook account, and one-third (107 million) have an Instagram account.8 9 Americans spend 53.5 billion min per month, or 38 min per user, per day, on Facebook.10 Leveraging the reach of social media is therefore highly attractive, and several previous studies have shown that social-media-based CC campaigns reach more people, are completed more efficiently, and are less expensive.5–7
However, a question remaining unanswered is whether the populations reached by social media campaigns are representative of the community as a whole. The purpose of this report is to compare the age and gender demographics of individuals reached by a recent CC social media campaign for an Exception From Informed Consent (EFIC) trial with those of the general population in the same area.
Methods
The PHOXSTAT trial
The ‘Pilot Randomized Clinical Trial of the XSTAT Hemostatic Device in the Prehospital Setting’ (PHOXSTAT)11 aims to evaluate the feasibility of conducting a study of the XSTAT device (RevMedX, Wilsonville, OR), a syringe-like applicator which injects small sponges into wounds in non-compressible sites.12 XSTAT may be helpful in controlling hemorrhage from penetrating junctional injuries, particularly in the prehospital setting, but its effectiveness has not been tested in a clinical trial.13 Conducting a randomized clinical trial of the XSTAT device is challenging, and this pilot trial was designed to answer key methodological questions before embarking on a phase III effectiveness trial.
PHOXSTAT will be conducted in three locations (Birmingham, Alabama; Los Angeles, California; and Nashville, Tennessee), by level I trauma centers and associated Emergency Medical Service agencies. Due to the potentially life-threatening injuries encountered during the trial and the inability to obtain informed consent, PHOXSTAT will be an EFIC trial.
The University of Alabama at Birmingham (UAB) is the lead institution of the PHOXSTAT trial, and its IRB was therefore selected as the central IRB for the study. UAB has extensive experience of using social media to conduct CC for EFIC trials,6 7 14 ,15 ,16 and after discussion with the IRB, this approach was again approved for use in this trial. The campaigns were administered by the Center for Injury Science at UAB, for all three sites. No additional (traditional or in-person) methods of CC were used. We have previously reported on the results of this campaign, in terms of the population reached and our ability to conduct social media based CC campaigns for multiple trial sites.14 17 The UAB IRB approved enrollment on the basis of the CC.
Social media campaigns
We created advertisements on Facebook (www.facebook.com) and Instagram (www.Instagram.com). Clicking on the advertisements forwarded users to study specific websites containing information about the trial, the reason it is being conducted, an explanation of EFIC regulations, and how to opt-out of this type of research. The websites also provided contact information for the Principal Investigator’s office, and allowed visitors to leave comments or submit questions, using an email form embedded in each website.
Communities
We targeted our social media campaigns to the approximate catchment areas of the participating trauma centers. For Birmingham and Nashville, we aimed our campaigns at individuals over the age of 18 within a 50-mile radius of UAB and Vanderbilt University Medical Center (VUMC), respectively, representing estimated populations of 1.6 and 2.3 million. The advertisements for these regions ran from March 24, 2020 to May 25, 2020. For Los Angeles, we targeted individuals of the same age, within a 20-mile radius of the Los Angeles County/University of Southern California trauma center (LAC+USC). The smaller radius—although it contains a larger population—was selected to distinguish the catchment area of this trauma center from the other level I trauma centers in Los Angeles. The advertisement for this area ran from June 22, 2020 to August 23, 2020.
Evaluation of social media reach
We evaluated the social media advertisements by examining user statistics provided by Facebook and Instagram. ‘Reach’ is a term used by Facebook and Instagram to describe the number of people who saw the advertisement at least once.18 Facebook and Instagram also provided information on the age and gender of individuals who had seen the advertisements. We analyzed the data using descriptive statistics.
Evaluation of population demographics
We conducted a geographic analysis of U.S. Census Bureau data to obtain the age and gender distribution of the population resident within the catchment areas described above. We used QGIS, a free and open-source cross-platform desktop geographic information system application (https://qgis.org/en/site/) to construct circular ‘buffers’ around each of the three trauma centers. We then analyzed the included census tracts, in terms of the gender and age distribution of the population. We then compared the age and gender distribution of the population as a whole with the population reached by our social media advertisement campaigns.
Results
Our advertisements reached a total of 332 081 individuals across all three sites, as reported in our previous publication.14
Table 1 shows the demographic breakdown of the population reached, by age group and gender, for each of the three sites.
Demographics of population reached
We reached 36 352 males and 33 456 females in the Birmingham area, 40 066 males and 37 024 females in the Nashville area, and 120 640 males and 60 416 females in the Los Angeles area. In all three sites, the proportion of individuals reached was broadly similar. We did not include social media users with unknown gender status in the further analysis, as there is no complementary category in the census data.
Table 2 contains details of each community’s population, again broken down by age and gender.
Demographics of resident population
There were 1.2m residents within a 50-mile radius of UAB, 1.7m residents within a 50-mile radius of VUMC, and 4.9m residents within 20 miles of LAC+USC. There were variations between and within each of the three study areas. The table also shows the proportion of males and females in each age group. The proportions of residents within each age group were similar across all three sites.
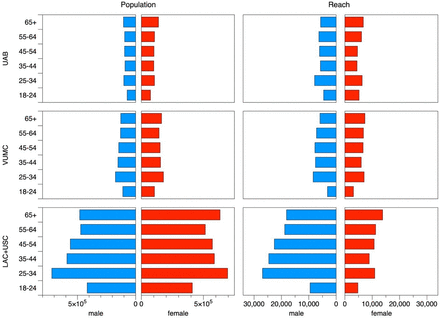
Figure 1 juxtaposes the age and gender structure of each area’s community (left hand column) and the population reached by our social media campaigns (right hand column). The area centered on LAC+USC medical center site had a larger population than the two other trial locations, but with broadly similar age and gender structure.
Number of residents in the areas centered on the three trauma centers (left side), and reached by social media campaign (right side). Results are broken down by gender (males, blue; females, red). LAC+USC, Los Angeles County + University of Southern California Medical Center; UAB, The University of Alabama at Birmingham Hospital; VUMC, Vanderbilt University Medical Center.
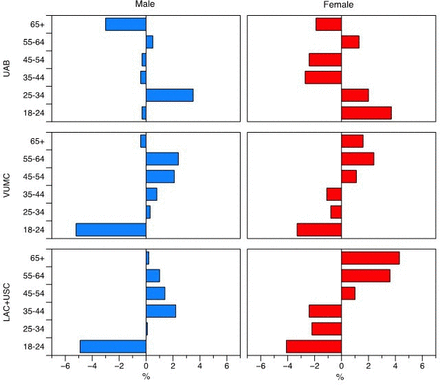
Figure 2 shows the differences in the proportions of individuals within each age group. For example, in Los Angeles, participants 18 to 24 years old made up 12.8% of the male population (table 1). Of all the males that were reached by the social media campaign, participants 18 to 24 years old accounted for 7.9% of the total sample (table 2). The difference between the community population and those reached by our CC is −4.9% (table 1), suggesting under-representation of this population segment.
Difference in proportion of individuals in each age group, and reached by CC campaign, by location, and gender. Bars pointing to the left of the center line indicate relative under-representation (a smaller proportion of individuals in this age group were reached by the social media campaign, compared with the population resident in this area). Bars pointing to the right indicate relative over-representation (a larger proportion of individuals in this age group were reached by the social media campaign, compared with the population resident in this area). CC, community consultation; LAC+USC, Los Angeles County and University of Southern California Medical Center; UAB, The University of Alabama at Birmingham Hospital; VUMC, Vanderbilt University Medical Center.
Similarly, in Birmingham, participants 18 to 24 years old accounted for 11.8% of the female population (table 2). Of all the people that were reached by social media advertising, participants 18 to 24 years old accounted for 15.5% of females (table 1). The difference in reach is 3.7%, suggesting over-representation of this population segment.
Overall, the individuals reached appeared to be broadly representative of each community, although there were small differences (within 5%) in representation in the three cities, in different age groups. In Birmingham, participants 55 to 64 years old, 25 to 34 years old, and females 18 to 24 years old were slightly over-represented in the sample of individuals that we reached by social media. In contrast, participants 35 to 54 years old, 65 and older, and males 18 to 24 years old were under-representation of this population segment (figure 2).
In Nashville, participants 18 to 24 years old and 65 and older were under-represented, whereas male participants 25 to 64 years old were over-represented in our sample. Among females in Nashville, those aged 45 years old and older were slightly over-represented in our sample, and those participants 18 to 44 years old were slightly under-represented in our campaign.
A similar pattern was seen in Los Angeles: again, females 45 years old and older were over-represented, and those participants 18 to 44 years old were under-represented in our sample. Male participants 18 to 24 years old were under-represented, whereas all other age groups were over-represented.
Discussion
The purpose of CC is to engage representatives of the community where the proposed trial will be conducted. The results of this study demonstrate that our social media-based CC campaign reached a sample which is broadly representative of the community as a whole, in terms of age and gender. This finding is of key importance to investigators and Institutional Review Boards when reviewing the results of social media-based CCs.
There are differences between age-groups and gender-groups, and between the three trial sites, but they are small, and probably of little consequence as far as the objective of consulting with the community is concerned. There are patterns, but these are not clear-cut. The most consistent is of a degree of under-representation of young women in Nashville and Los Angeles, and over-representation of older women in these locations. However, this pattern is almost reversed in Birmingham. Similarly, there is under-representation of 18 to 24-year-old men in Los Angeles and Nashville, and a degree of over-representation among the middle-aged. This pattern, again, differs in Birmingham. This study was not designed to elucidate the reasons for these subtle variations. It is conceivable that these location-specific patterns reflect demographic differences in social media use, or idiosyncrasies related to the social media platforms’ algorithms for selecting individuals to whom the advertisements are sent.
Traditional methods of CC, such as public forums, town halls, and print, radio, and TV adds, random digit dialing, are often regarded as the ‘gold standard’ against which social media-based CC is compared. However, these methods reach far fewer individuals than social media campaigns do. A recent article analyzed the number of people reached with traditional, in-person CC approaches, in the Houston area, for three EFIC trauma trials.5 The trials each required 12 to 14 meetings and made contact with an average of only 243 individuals. This number represents a tiny fraction of the 69 808 people which our campaign reached in the Birmingham area, and the 77 090 and 181 056 people reached in the Nashville and Los Angeles areas, respectively.5
This study has limitations, and there are unanswered questions. The demographic data provided by social media users may not be accurate. The variations observed across age-groups and gender-groups, and locations, although small, are worth exploring in future CC campaigns, especially those involving more than three centers. Race and ethnicity of users is another area of interest, although such data are difficult to obtain from social media companies. Last, having received a notification of a trial on Facebook is not informative in itself—but it does indicate that the opportunity to engage has been provided. Social-media-based consultation is no different from receiving a mailer, and throwing it away without looking at it; or not going to a town hall meeting that was advertised; or declining a call to inform about the trial.
Conclusion
In conclusion, this study demonstrates that CC campaigns conducted through social media can be used to reach a sample of the community broadly representative of the population as a whole, at least in terms of age and gender. This finding is helpful to IRBs and investigators, as it lends further support to the use of social media to conduct CC. Further work is needed to analyze how representative community samples are in terms of other characteristics, such as race, ethnicity, and socioeconomic status.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Ethics approval
This study was conducted under the PHOXSTAT IRB at UAB approval IRB-300003647 which is an Exception From Informed Consent trial.
Footnotes
Contributors SWS, KNB, and JOJ created the social media campaign. SWS designed and ran the social media advertisements and study websites. SWS, PF, and JOJ analyzed the results. SC, BC, ABP, BMD, NR, KI, and JBH assisted with the interpretation of the results. All authors contributed to the writing of the article. JOJ is the guarantor.
Funding The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. This work was supported by the Department of Defense through the U.S. Army Medical Research Acquisition Activity, under Award No. W81XWH-16-R-BAA1. JJ is a consultant for Cellphire and CSL Behring. He has grants with the NIH, NIHR, and DoD. JJ has industry support through Infrascan, CSL, Behring, RevMedX, and Prytime. JBH is a consultant for Arsenal Medical, Cellphire, Safeguard, and Medical Device International. He is on the board of directors for Zibrio, QinFlow, and Decisio Health. He is a cofounder for Decisio Health and a coinventor of the Junctional Emergency Tourniquet Tool for which he receives loyalties from UT Health.
Disclaimer Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the U.S. Army Medical Research and Development Command.
Competing interests JJ is a consultant for Cellphire and CSL Behring. He has grants with the NIH, NIHR, and DoD. JJ has industry support through Infrascan, CSL, Behring, RevMedX, and Prytime. JBH is a consultant for Arsenal Medical, Cellphire, Safeguard, and Medical Device International. He is on the board of directors for Zibrio, QinFlow, and Decisio Health. He is a cofounder for Decisio Health and a coinventor of the Junctional Emergency Tourniquet Tool for which he receives loyalties from UT Health. The other authors have no conflicts of interest to report.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.