Abstract
Background Approximately 8% of traumatically injured patients require transfusion with packed red blood cells (pRBC) and only 1% to 2% require massive transfusion. Intraoperative massive transfusion was defined as requiring greater than 5 units (u) of pRBC in 4 hours. Despite the majority of patients not requiring transfusion, the appropriate amount and type of crystalloid administered during the era of damage control resuscitation have not been analyzed. We sought to determine the types of crystalloid used during trauma laparotomies and the potential effects on resuscitation.
Methods Patients who underwent laparotomy after abdominal trauma from January 2014 to December 2016 at the University of Cincinnati Medical Center were identified. Patients were grouped based on requiring 0u, 1u to 4u, and ≥5u pRBC during intraoperative resuscitation. Demographic, physiologic, pharmacologic, operative, and postoperative data were collected. Statistical analysis was performed with Kruskal-Wallis test and Pearson’s correlation coefficient.
Results Lactated Ringer’s (LR) solution was the most used crystalloid type received in the 0u and 1u to 4u pRBC cohorts, whereas normal saline (NS) was the most common in the ≥5u pRBC cohort. Most patients received two types of crystalloid intraoperatively. NS and LR were most frequently the first crystalloids administered, with Normosol infusion occurring later. The amount of crystalloid received correlated with operative length, but did not correlate with the estimated blood loss. Neither the type of crystalloid administered nor the anesthesia provider type was associated with changes in postoperative resuscitation parameters or electrolyte concentrations.
Discussion There is a wide variation in the amount and types of crystalloids administered during exploratory laparotomy for trauma. Interestingly, the amount or type of crystalloid given did not affect resuscitation parameters regardless of blood product requirement.
Level of evidence Level IV.
Introduction
There were almost 21 million inpatient discharges following traumatic injury across the USA with an increasing annual cost from $12.0 to $29.1 billion from 2000 to 2011.1 Although hemorrhage remains the leading cause of potentially preventable death after trauma in both the civilian and military populations, only a select minority of patients require blood transfusions.1–6 It is estimated that only 8% of civilian trauma patients require transfusion with packed red blood cells (pRBC) and only 1% to 2% of patients require massive transfusion.5 6 Much research has been undertaken to improve resuscitation, including the advent of damage control resuscitation (DCR) strategies.2 5 7–10 The authors of the Prospective, Observational, Multicenter, Major Trauma Transfusion study developed a tool, resuscitation intensity (RI), as a marker of overall physiologic derangement in bleeding patients that was associated with increased mortality. The RI takes into account all blood products, crystalloid, and colloid used during the initial resuscitation.11 Current resuscitation techniques and DCR focus on the prevention of “lethal triad” of hypothermia, acidosis, and coagulopathy, thus reducing mortality.5 10 12 13
Salt-containing intravenous fluid solutions have been a mainstay of resuscitation strategies during the last 50 years. Massive intravenous fluid administration, however, has been associated with hypothermia, acute respiratory distress syndrome, hyperchloremic metabolic acidosis, anastomotic leakage, prolonged ileus, multiorgan dysfunction, dilutional coagulopathy, and mortality.2 12 14–16 Excess fluid administration has been shown to decrease tissue oxygenation, increase tissue edema, increase cellular apoptosis, activate neutrophils, and increase circulating proinflammatory cytokines.16 17 When comparing normal saline (NS) administration with lactated Ringer’s (LR) solution, patients receiving NS demonstrate greater hyperchloremic acidosis and dilutional coagulopathy, and require higher volumes to accomplish resuscitation in the setting of hemorrhagic shock.18 19 These derangements have been demonstrated after as few as 3 L of crystalloid administration.17
In non-trauma literature, recent research has favored the use of a balanced crystalloid (LR and Normosol) over NS. The composition of the fluids varies greatly and likely has contributed to the shift toward more balanced crystalloids: NS (154 mEq/L NaCl, pH 5.5), LR (130 mEq/L Na+, 109 mEq/L Cl−, 4 mEq/L K+, 2.7 mEq/L Ca2+, and 28 mEq/L lactate, pH 6.5), and Normosol (140 mEq/L Na+, 98 mEq/L Cl−, 5 mEq/L K+, 3 mEq/L Mg2+, 27 mEq/L acetate, and 23 mEq/L gluconate, pH 7.4). The use of NS has been associated with a decrease in renal blood flow and perfusion in healthy volunteers.20 Furthermore, an increase in morbidity and mortality has been observed in patients with systemic inflammatory response syndrome and patients undergoing non-emergent laparotomy if the predominant resuscitative fluid is NS, compared with a calcium-free crystalloid.21 22 By contrast, the use of balanced crystalloids has demonstrated decreased mortality, need for renal replacement therapy, and continued renal dysfunction in critically ill and non-critically ill patients as compared with NS utilization.23 24
To our knowledge, there have been no studies evaluating the types of crystalloid resuscitation administered in the operating room during exploratory laparotomy for acute traumatic injury. In this analysis, we sought to identify (1) which crystalloids were used most commonly during trauma laparotomy, (2) whether transfusion amounts and estimated blood loss (EBL) were associated with the amount and type of crystalloid used, and (3) whether crystalloid selection affected markers of resuscitation.
Patients and methods
After Institutional Review Board approval from the University of Cincinnati, a retrospective review of all patients who underwent emergent laparotomy for acute abdominal trauma between January 2014 and December 2016 were identified from the prospectively maintained University of Cincinnati Medical Center trauma registry. Only patients who went directly from the emergency department to the operating room for primary laparotomy were included in the data analysis. All intraoperative deaths and delayed laparotomies were excluded from analysis to allow for a more focused investigation on intraoperative management that may affect postoperative recovery. Intraoperative resuscitation was primarily directed by the anesthesia team, guided by patient physiology, and assisted only by the institutional massive transfusion protocol when appropriate; otherwise, there are no formalized protocols in place at our institution.
Patient demographics, physiologic parameters, and injury characteristics were collected from the trauma registry and electronic medical records. Analyzed data included patient age, gender, mechanism of injury, postinjury hospital transfer rate, body mass index (BMI), Injury Severity Score (ISS), postoperative complications, operative time, intraoperative EBL, anesthesia provider (resident or certified registered nurse anesthetist [CRNA]), overseeing anesthesia attending, first preoperative and postoperative heart rate (HR) and systolic blood pressure (SBP), first preoperative and postoperative pH, base excess (BE), hematocrit (Hct), lactate, chloride (Cl−), glomerular filtration rate (GFR), and sodium (Na+). Intraoperative fluid volumes, types, and timing of each crystalloid and colloid administered were collected from the anesthesia record. The volumes and types of blood products were extracted from the anesthesia record, operative report, and blood bank reports. Data on the vasopressors used were collected from the anesthesia record. The RI was calculated based on previously described methods, as defined by the total number of intraoperative units of crystalloid, colloid, and blood products administered.11 One unit was defined as 1000 mL crystalloid, 250 mL hypertonic saline, 500 mL colloid, or 1 unit of blood product.
Patients were identified as receiving 0 units (u) of pRBC, 1u to 4u of pRBC, or ≥5u of pRBC during their operative intervention. Given that many patients do not require pRBC resuscitation, our cohorts were determined to represent three distinct patient populations. The 0u pRBC cohort represents the majority of our patients and only require crystalloid resuscitation. The 1u to 4u pRBC cohort represents patients who are bleeding but do not require massive transfusion. Patients requiring ≥5u of pRBCs represented a massive transfusion group as most cases were completed within 4 hours.25 The primary outcome was the correlation between the amount of crystalloid received and EBL. The secondary outcomes included correlation between the amount of crystalloid and RI, correlation between the amount of crystalloid and units of pRBC, and correlation between the amount of crystalloid and different types of crystalloid. We also examined the correlation between the amount of each crystalloid administered and subsequent resuscitation parameters (pH, sodium, lactate, Cl−), as well as the correlation between the type of anesthesia provider, fluid administered, and the resulting end resuscitation parameters.
GraphPad Prism V.7 was used for statistical comparisons (GraphPad Software, La Jolla, CA). Continuous data are presented as mean±SD or median (IQR) based on value distribution testing, and analyzed by analysis of variance with Holm-Sidak test or Kruskal-Wallis with Dunn’s test where appropriate. Categorical data are presented as numbers or percentages and were analyzed using the χ2 test. Pearson’s product-moment coefficient analysis was performed to determine correlation. A correlation coefficient of ≤0.35 was considered a weak correlation, a moderate correlation demonstrated a correlation coefficient from 0.36 to 0.67, and a value of ≥0.68 was considered a strong correlation.26 A p<0.05 was considered significant.
Results
During the 36-month study period, a total of 504 patients underwent emergent laparotomy; 42 patients were excluded due to intraoperative death or delayed laparotomy. Of the 462 analyzed patients, 237 (51%) received 0u pRBC, 143 (31%) received 1u to 4u pRBC, and 82 (18%) received ≥5u pRBC. All laparotomies included a board-certified surgeon and anesthesiologist during initial intraoperative resuscitation. Each anesthesiologist performed a median of 7 (2, 18) trauma laparotomies during the study period. Age, BMI, postinjury hospital transfer rate, operative duration, and fluid administration rate were similar between groups (table 1). Patients requiring 1u to 4u pRBC sustained a higher proportion of blunt trauma, and the population had a higher percentage of female individuals as compared with the 0u pRBC and ≥5u pRBC cohorts. Not surprisingly, EBL, ISS, and RI were significantly greater in the cohorts requiring more pRBC transfusions. However, there were no differences in the duration of operation or total crystalloid administered. The percentage of crystalloid used in the total RI was significantly different between cohorts, with crystalloid constituting only 11% of the total RI in the ≥5u pRBC cohort.
Demographic data
Patients requiring more pRBC transfusions demonstrated worsened preoperative HR, SBP, pH, BE, lactate, Hct, and GFR, indicative of a more profound state of hemorrhagic shock (table 2). Perioperatively, both the 1u to 4u and ≥5u pRBC cohorts demonstrated significantly higher vasopressor requirements, used more than one vasopressor agent, and in the ≥5u pRBC cohort had a higher proportion of patients leaving the operating room on vasopressors. Postoperatively, the 1u to 4u pRBC cohort demonstrated a significant decrease in GFR and Hct compared with patients who did not receive pRBC transfusion; no other parameters were statistically significant. The ≥5u pRBC cohort also demonstrated significant differences in postoperative resuscitation markers compared with the 0u pRBC and the 1u to 4u pRBC cohorts (table 2).
Resuscitation markers
The most common fluid administered and thereby the largest contributor to total crystalloid volume in the 0u pRBC and the 1u to 4u pRBC cohorts was LR, with 85% and 80% receiving LR, respectively (table 3). NS was the most commonly used fluid in the ≥5u pRBC cohort, with 78% of patients receiving NS. There were significant differences in the pRBC, platelet, fresh frozen plasma (FFP), and albumin resuscitation between cohorts; no other types of colloid were administered intraoperatively during the study period. There was no difference in the total amount of crystalloid received or in the order in which the fluids were administered. The median number of different crystalloids used was 2 in all cohorts. Both NS and LR were used concurrently at the start of the operative case in 27%, 34%, and 28% in the 0u pRBC, 1u to 4u pRBC and ≥5u pRBC cohorts, respectively. Normosol was less likely to be the first crystalloid administered and was started at a median of 55, 65, and 70 minutes into the operative case in the 0u pRBC, 1u to 4u pRBC and ≥5u pRBC cohorts, respectively.
Intraoperative fluid administered
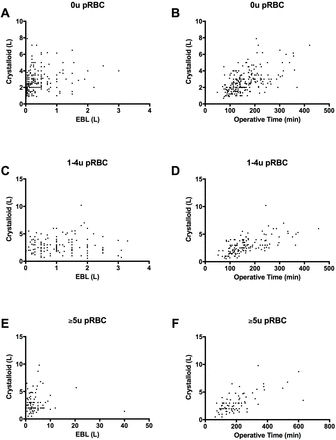
There was no correlation between EBL and volume of crystalloid resuscitation in any of the cohorts (figure 1). Moderate correlation was demonstrated in all cohorts when comparing operative time and volume of crystalloid given, suggesting that the longer a patient was in the operating room, the more fluids they received. Strong correlation was demonstrated in the 0u pRBC cohort when comparing crystalloid volume with RI, but this was only moderate in the 1u to 4u pRBC cohort, and no correlation was demonstrated in the ≥5u pRBC cohort (table 4). There was a moderate correlation in all groups between the crystalloid volume and the number of crystalloids given in all cohorts, suggesting that crystalloid selection changed to favor more balanced crystalloid use as more fluid was administered. There was no correlation between the amount of each fluid selected and the subsequent fluid given, or postoperative pH, Na+, GRF, and lactate. There was mild positive correlation between Cl− concentration and the percent of NS administered, and mild negative correlation between Cl− concentration and the percent of LR administered, although this was only observed in the 0u pRBC cohort.
Correlation analysis
Correlation between the volume of crystalloid administered vs. estimated blood loss (L) (A, C, E) or operative time (B, D, F). Patients requiring 0u pRBC (A and B, n=237, r=0.247 [p<0.05] and r=0.558 [p<0.05], respectively), patients requiring 1u–4u pRBC (C and D, n=143, r=0.047 and r=0.619 [p<0.05], respectively), and patients requiring ≥5u pRBC (E and F, n=82, r=0.041 and r=0.666 [p<0.05], respectively). pRBC, packed red blood cells; u, unit.
When analyzing fluid administration by provider, either resident, CRNA, or resident and CRNA, there was again a moderate correlation in all groups between crystalloid volume and operative time, and between crystalloid volume and number of different crystalloids (table 5). Mild correlation was demonstrated in the ≥5u pRBC cohort between crystalloid volume and RI. A positive mild correlation existed in the ≥5u pRBC cohort between percent NS and Cl−, and percent NS and Na+. A negative mild correlation was demonstrated between percent LR and Cl−, and percent LR and Na+ in the ≥5u pRBC cohort. No other correlation existed between the percent of fluid composition for each type of fluid and other resuscitation parameters. Supporting anesthesiologist (resident, CRNA) was not associated with differences in fluid type or amount administered.
There were no differences or correlations between variables when the data for each cohort were analyzed in quartiles by the volume of crystalloid or total percent NS (data not shown). The correlations between intraoperative crystalloid types and acute respiratory distress syndrome, wound infection, compartment syndrome, decubitus ulcer, pneumonia, acute renal failure, and sepsis complications from our Trauma Quality Improvement Program were analyzed and no significant moderate or strong correlations were observed.
Provider correlation analysis
Discussion
To our knowledge, this is the first study that has observed the impact of intraoperative crystalloid resuscitation during emergency exploratory laparotomy in the trauma population. Although there has been a large amount of research published concerning the effects of crystalloid on human physiology, morbidity and mortality, there is a lack of data within the trauma population. Here, we have shown that there is a wide distribution of the type and volume of intraoperative crystalloid administered during trauma laparotomy. A moderate correlation was demonstrated between the amount of crystalloid given and the time in the operating room, but no correlation was seen when comparing crystalloid volume and EBL or pRBC administration. Furthermore, no correlation was demonstrated between the crystalloid used as the primary resuscitative fluid and end resuscitation markers. In addition, the type of anesthesia provider did not have an impact on the amount of crystalloid, type of crystalloid administered, or postoperative resuscitation markers.
The patients were stratified based on transfusion requirements to examine discrete patient populations, as it would be expected that patients in more severe hemorrhagic shock would receive more blood products and less crystalloid volume. Patients requiring ≥5u of pRBCs represented a massive transfusion group as most cases were completed within 4 hours.25 As up to 92% of trauma patients do not receive blood products, we found it important to study patients who did not require transfusion to represent the majority of the trauma population.6 Our aim was to stratify patients in a way that was most natural to the progression of trauma patients being admitted to the emergency department and expediently taken to the operating room. Most of the information available at this time would consist of laboratory values and clinically based on patient hemodynamic status.
Given DCR strategies, it was not surprising that there were significant differences in the ISS, RI, EBL, and resuscitation markers, as these populations were defined by the amount of blood products received. Overall, the blood product resuscitation at our institution followed the optimal 1:1 FFP:pRBC strategy, which has demonstrated increased survival.27 Colloids were used during the resuscitation of all groups, but were given to a higher proportion of the ≥5u cohort. Although colloids have often been thought of as a superior resuscitative fluid to crystalloids, no studies have demonstrated a clear mortality benefit, reduction of blood transfusion, or need for renal replacement therapy.28
As expected, patients who required more transfusions had a higher ISS, required more blood-based resuscitation, required more vasopressor support, and had more metabolic derangements on arrival and exit from the operating room. All cohorts showed a trend toward improvement based on postoperative resuscitation makers, demonstrating ongoing resuscitation with crystalloid and/or blood products. There was also a significant decline in GFR at the end of the case in the cohorts requiring more transfusion. This was likely due to the severity of shock, as prior research has demonstrated the development of acute kidney injury following hypotension and hypoperfusion.29 The effect of vasopressors on renal function has largely shown increased renal perfusion, but the results are mixed, and in our study did not suggest renal protection.30
Most patients received two types of crystalloid during laparotomy, with NS and LR most often administered simultaneously at the beginning of the case. This was likely due to anesthesia providers choosing LR to be the primary resuscitative fluid but requiring NS to be administered with pRBC due to the concern that calcium could chelate the anticoagulating citrate and clot the pRBC within the intravenous line. When Normosol was used, it was only after the administration of NS and/or LR, with the transition to Normosol occurring after approximately 1 hour of operative time. There have been many publications suggesting that balanced crystalloids are a superior choice of resuscitative fluid due to a decreased morbidity and mortality in critically ill, non-critically ill, and non-emergent surgical patients.21–24 Self et al 24 demonstrated a lower incidence of persistent renal dysfunction and new renal replacement therapy in non-critically ill patients receiving Normosol as compared with NS. Semler et al 23 demonstrated a decreased rate of death, persistent renal dysfunction, and new renal replacement therapy in critically ill patients receiving Normosol as compared with NS. The major limitations in comparing these studies remain the population; Shaw et al purposefully excluded the trauma population, and Semler et al and Self et al included all patient populations (medical and surgical). This is the first study to look specifically at only the trauma population and specifically those undergoing emergency laparotomy.
The majority of patients in this study received NS as part of their intraoperative resuscitation, whereas only a minority of patients received Normosol. A mild correlation between the amount of NS received and Cl− concentration was demonstrated in some cohorts, but was not consistent. No correlation was seen between increased NS use and acidosis immediately postoperatively. In the literature, this relationship has been described, with increased NS administration resulting in hyperchloremic acidosis.14–16 18 19 However, as most patients received both NS and LR—often times simultaneously—the effects of the more balanced LR may have mitigated the negative consequences associated with higher volumes of NS administration. Given the emergent and often severe physiologic derangements, immediate postoperative laboratory values may have also been skewed by the use of intraoperative pharmacologic therapies. We also were unable to demonstrate superiority or inferiority of one type of fluid over another when comparing postoperative resuscitation markers. This was again likely confounded by the use of multiple crystalloid types during the operation in the majority of patients. There are limited available data or ability to ascertain retrospectively why a shift in crystalloid type was made during each case, but potential explanations may include fluid availability/convenience, acid/base status, clinical state, which fluid was started in the ED, or provider bias.
Although the amount of crystalloid received correlated with the amount of time spent in the operating room, we did not observe any correlation between EBL or pRBC and crystalloid volume. Despite increased blood loss in the ≥5u pRBC cohort, operative times and fluid administration per hour were similar. All cohorts received a median of 1 L/hour (or 10–11 mL/kg/hour) of crystalloid regardless of ongoing resuscitation with blood product with a total median volume of 2.5 L to 2.7 L administered in all cohorts. Although one might expect a positive correlation, there has been a paradigm shift in fluid resuscitation from a liberal to a more restricted approach, although no strong evidence has been demonstrated and there is renewed interest in intraoperative fluid resuscitation.31–37 Shin et al have recently demonstrated optimal postoperative outcomes are associated with crystalloid infusion rates of approximately 6 to 7 mL/kg/hour or 1 L of fluid for a 3-hour operative case in an non-emergent setting with minimal blood loss.36 Myles et al recently demonstrated that a restrictive resuscitation (median crystalloid volume of 1.7 L) was associated with a higher rate of kidney injury and no difference in disability-free survival as compared with a liberal resuscitation (medial crystalloid volume of 3.0 L).37 Although in our institution crystalloid appears to be given as a lower percentage of the total resuscitation as RI increases, our providers do not appear to reduce hourly administration of crystalloid across our three transfusion cohorts. Whether this level of hourly crystalloid administration is harmful for patients in any of these cohorts remains to be determined.
Within the trauma population, multiple studies have demonstrated the detrimental effects of large crystalloid volumes, specifically in those patients requiring massive transfusion.12–18 38 There remains a knowledge gap for those patients not receiving blood products at all. More specifically, however, trauma patients present unique physiologic changes compared with elective operative cases; they are more likely to arrive hypovolemic, acidotic, coagulopathic, and hypothermic.3 5 8 This presents unique challenges in managing resuscitation. Massive transfusion protocols have guided the actively hemorrhaging patients and have decreased morbidity and mortality.2–5 7 8 No protocols/guidelines have been developed to aid in the trauma population that are not actively hemorrhaging but require emergent operative intervention.
Each anesthesia attending oversaw/performed a median of 7 trauma laparotomies during the study design, which averages to only 2 to 3 cases per year. During each of these cases, there is also variability in the assisting provider based on resident and CRNA involvement. Multiple studies have demonstrated significant provider variability across individual anesthesia provider, surgical specialties, and regional hospitals.36 38–40 Lilot et al demonstrated wide interprovider and intraprovider variability when departmental fluid administration guidelines were not in place. They determined that most providers are inconsistent in their individual approaches based on a wide range of corrected volumes administered, and overall the volume of crystalloid administered is largely based on the individual giving the fluid and not based on patient or procedural factors.39 It has been suggested that protocols should be developed and rigorously implemented based on widespread provider variability in non-emergent abdominal operative intervention.36 39–41 Given the limited number of cases each anesthesia provider performs in a year, we agree that there may be a role for standardization of crystalloid usage in the intraoperative trauma population.
This study is limited in being a retrospective review, which comes with its own inherent difficulties. It is also a single institution review, which may have limited applicability to the global trauma population and other institutions. There is also concern for anesthesia provider bias during intraoperative resuscitation, as a small group of attendings performed the resuscitation on a higher number of cases. Given that our cohorts were intentionally different in the amount of pRBCs administered, the results may also be confounded by the use of blood products and other pharmacologic adjuncts used during massive transfusion, leading to improved resolution of hemorrhagic shock. Our results may also be skewed as the majority of patients received both NS and LR at the start of the operation instead of a single intravenous fluid throughout each case. However, this highlights an important point: there does not appear to be a standard choice in resuscitation fluid by anesthesia providers at our institution.
Based on the results of this study and following discussions with our own anesthesia department, we sought to determine why our fluid resuscitations seemed to be primarily NS-driven and with multiple types of crystalloids. It was discovered that anesthesia technicians set up the trauma operating room with NS as the default fluid for anesthesia providers to use. Normosol was historically regarded as cost prohibitive in our institution, but after further review it was determined that the cost is currently equivalent for all three crystalloids. An institutional change was made to have Normosol available as the primary resuscitative fluid to anesthesia providers. This change was based on the literature in non-trauma patient populations demonstrating the superiority of a balanced crystalloid fluid, the ability of Normosol to be infused with blood, and the decreasing cost of Normosol.19–24 Nevertheless, there remains a need for multi-institutional clinical trials to further evaluate the effects of the type of crystalloid resuscitation during trauma laparotomies and determine the ideal resuscitative crystalloid. A study mirroring the Vanderbilt group, in which the intravenous fluid administered is controlled from the emergency department to discharge, would be of value to identify the ideal crystalloid.23 24 Furthermore, future studies are needed to determine the cost of using a Normosol-derived intraoperative resuscitation protocol as compared with an unprotocolized resuscitation strategy.
Conclusions
This is the first study to specifically look at intraoperative fluid administration during exploratory laparotomy for trauma. A wide variation in the amounts and types of crystalloids administered intraoperatively was encountered. Whereas there was a correlation between the amount of crystalloid administered and operative length, there was no correlation with EBL or pRBCs given. Furthermore the type of crystalloid administered and anesthesia provider did not have an effect on postoperative resuscitation markers. This is the first study to specifically look at intraoperative fluid administration during emergent exploratory laparotomy for trauma. Although there was a trend toward a more balanced crystalloid fluid as the operation progressed, there remains a large gap in understanding how best to optimize the intravenous fluid administration to the trauma patient, and further prospective multicenter studies are needed.
Acknowledgments
The authors would like to thank Lita Holdeman, the trauma coordinators and the registry members responsible for the acquisition and maintenance of the University of Cincinnati Trauma Registry.
Footnotes
Presented at Presented at the Academic Surgical Congress, February 2018, Jacksonville, Florida.
Contributors JEB, GEM, SAJ, VN, ATM, MDG contributed to the design of the study. JEB, GEM, GK, CJW contributed to the acquisition of data. JEB, GEM, GK, CJW, MDG contributed to data analysis. JEB, GEM, HVL, MDG drafted the article. All authors contributed to critical review of the article and have agreed to the final version of the article.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval University of Cincinnati Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
This is an Open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.