A 27-year-old man presented to the trauma center with a close-range shotgun wound to the proximal right thigh.
Examination
The patient was awake and alert with a heart rate of 120 beats per minute, a systolic blood pressure of 80 mm Hg, and a base deficit of −10. The pressure dressing over a 7 cm diameter blast cavity in the mid-anterior proximal right thigh was saturated with blood and replaced. No exit wound was noted, and no arterial pulses were present in the right foot.
Management
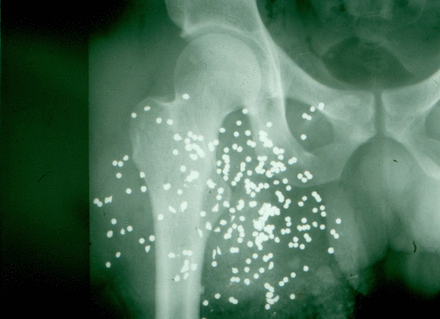
As a blood specimen was drawn for type and crossmatch, resuscitation with crystalloid solutions was initiated (no massive transfusion protocol available at the time). The X-ray of the right groin and thigh documented that almost all pellets were in the thigh itself (figure 1). After administration of a cephalosporin antibiotic, the patient was moved to the operating room.
Shotgun wound to the proximal right thigh.
Skin preparation and draping was from the umbilicus to the bilateral toenails, and the right foot was placed in a plastic bag. The open shotgun wound was too proximal to allow for the placement of a tourniquet or blood pressure cuff, so continuous pressure was applied. An 8 cm longitudinal incision in the right inguinal area was made proximal to the area of the shotgun wound. The right common femoral artery was exposed and an angled DeBakey vascular clamp was applied. A vessel loop was placed around the right common femoral vein as well. A separate 8 cm longitudinal incision was then made in the medial right thigh distal to the area of the shotgun wound. Both the superficial femoral artery and the femoral vein were clamped in this location as was the distal femoral vein in the groin.
The incisions were extended into the shotgun wound cavity. Multiple vessels in the blast cavity were clipped or ligated. It was quickly obvious that a 15 cm segment of the right superficial femoral artery 2 cm beyond the common femoral artery and a 15 cm segment of the adjacent femoral vein beyond the entrance of the greater saphenous vein were destroyed. The profunda femoris artery and vein were destroyed as well. The patient was remarkably stable at this point with vascular clamps on the proximal and distal superficial femoral artery and femoral vein. The remnants of the profunda femoris artery and vein were ligated. No unfractionated heparin was administered at this time.
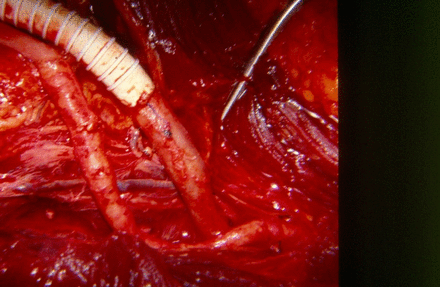
The insertion of interposition grafts in situ into the superficial femoral artery and femoral vein would place these grafts into the shotgun blast cavity, an unsatisfactory location. After two negative passes of a #4 Fogarty balloon catheter into both ends of the right superficial femoral artery, 25 mL of “regional” heparin (50 units/mL) was administered into each end. A reversed autogenous saphenous vein graft from the left thigh was then sewn end to end to the proximal stump of the right superficial femoral artery using 5–0 polypropylene suture. Then a ringed 8 mm polytetrafluoroethylene graft was sewn end to end to the distal stump of the right femoral vein using 6–0 polypropylene suture (figure 2).
Extra-anatomic saphenous vein bypass graft replacing the right superficial femoral artery and 8 mm ringed polytetrafluoroethylene graft replacing the right femoral vein. Note the blast cavity of the shotgun wound at the bottom of the figure. (With permission from Feliciano DV14).
The subcutaneous tissue and skin of the right thigh medial to the shotgun wound were elevated with an electrocautery. The saphenous vein graft was placed in this new extra-anatomic location. The distal end-to-end anastomosis to the superficial femoral artery was then completed with the last several loops of 5–0 polypropylene suture being left loose. The clamp on the proximal superficial femoral artery was removed to allow for flushing and then reapplied. The distal clamp was removed to flush the distal vessel and remove air from the system, the loose sutures were pulled tight, and one knot was in place when the proximal clamp was released. Palpable pulses were noted in the right ankle and foot. The 8 mm ringed polytetrafluoroethylene graft was then placed in a similar medial extra-anatomic position. The graft was sewn end to end to the proximal femoral vein in the mid-thigh using 6–0 polypropylene suture (figure 3).
Distal saphenous vein-to-superficial femoral artery anastomosis and proximal 8 mm ringed polytetrafluoroethylene graft-to-femoral vein anastomosis.
Suture closure of the subcutaneous tissue and skin proximally and distally fixated the medial extra-anatomic grafts at a distance of 4 to 10 cm from the shotgun blast cavity. The shotgun blast cavity was vigorously debrided and packed with fine mesh gauze. With daily dressing changes, the cavity filled in with a granulating surface (figure 4), and a split-thickness skin graft was applied on the 57th day after injury. There was 100% healing of the skin graft, and the patient was discharged on the 63rd day after injury on aspirin (ASA) 81 mg every 12 hours. When examined in the outpatient clinic on the 102nd day after injury, he had normal right pedal pulses and no peripheral edema (figure 5).
Soft tissue defect in the right thigh prior to application of a split-thickness skin graft.
Healed split-thickness skin graft on 102nd day after injury.
Discussion
Non-anatomic or extra-anatomic vascular bypasses were originally developed to treat prosthetic graft occlusion or infection after elective vascular operations over 65 years ago.1 A large number of bypasses were developed from 1951 to 1966, including those that are most familiar to vascular and general surgeons—femorofemoral (1952 and 1958), axillofemoral (1962), and obturator (1963).
These bypasses have been used in victims of civilian trauma since 1973, primarily in those with shotgun wounds to the extremities or postoperative graft infections in the brachial, iliac, or femoral arteries.2–12 The indications for an extra-anatomic bypass have remained the same over time as follows: (1) loss of soft tissue over injured artery or vein; (2) postoperative infection of the incision with blowout of underlying arterial repair; and (3) infections of both soft tissue and underlying native artery secondary to injection of illicit drugs.
In the patient described, undermining of the subcutaneous tissue of the injured thigh allowed for an extra-anatomic position of both grafts without creating a separate subcutaneous tunnel. This is not possible in most patients, and a carefully placed separate subcutaneous (or intermuscular) tunnel is a critical part of the operation. The operative technique has been comprehensively described in several recent publications, so only an abbreviated summary is presented as follows12 13:
The patient without a coagulopathy or residual oozing from the injury is systemically heparinized.
Healthy proximal and distal artery and/or vein is exposed through separate longitudinal incisions outside the area of injury, and injured vessels are excised back to this area. Fogarty balloon catheters are passed into the proximal and distal healthy artery until no clot has been retrieved on two separate passes. If systemic heparin has not been administered, 1000 to 1200 units of unfractionated heparin (20–25 mL of heparinized saline at 50 units/mL) are injected both proximally and distally. Both ends of the healthy vein are flushed.
Proximal arterial and/or distal venous vessel-to-graft anastomoses are completed with polypropylene sutures.
A black line is placed on the saphenous vein and/or ringed polytetrafluoroethylene (PTFE) graft to confirm proper orientation before passage through the new subcutaneous tunnel.
A Bakes dilator or long Kelly clamp is used to bluntly create a subcutaneous gently curved tunnel from the healthy proximal vessel around the area of injury to the healthy distal vessel.
The vascular graft is pulled through the subcutaneous tunnel using the black line for orientation.
Flushing of the arterial graft is performed through the distal open end; also, the desired length of the graft can be determined while it is pulsatile in the tunnel.
The proximal artery is reclamped above the anastomosis, the tunnel is compressed to evacuate residual blood from the graft, and a PTFE venous interposition graft can be flushed with heparinized saline.
The graft is shortened where marked and sewn in an end-to-end fashion to the distal healthy artery with 5–0 or 6–0 polypropylene suture. The last few loops of suture are left loose to allow for proximal flushing and the reapplication of a vascular clamp just proximal to the distal anastomosis. The distal clamp is removed for flushing and to remove air up to the adjacent proximal clamp.
The proximal clamp is partially removed to flush out any residual air and then fully removed after the first knot of the polypropylene suture is tied down.
Completion arteriography encompassing both arterial-graft anastomoses and the graft is performed through a 20-gauge cannula over metal needle placed in the proximal artery.
The incisions over both anastomoses are closed, and the soft tissue defect is vigorously debrided and packed.
ASA 81 mg every 12 hours is initiated 12 hours postoperatively and continued for 3 months.
One possible complication of this procedure is distal in situ arterial thrombosis secondary to hypotension, a prolonged operative time, and an unrecognized compartment syndrome below the knee. The passage of a Fogarty balloon catheter before the artery-graft anastomoses and passage of the graft through the tunnel should help with this problem. If arterial flow is once again sluggish prior to completion of the distal anastomosis, the Fogarty catheter should be passed proximally and distally again.
Another possible complication is failure to adequately cover the artery or vein-to-graft suture lines. Resection to normal vessel should be 5 to 6 cm away from the soft tissue defect to allow for easy complete coverage of the anastomoses as the graft enters the gently curving tunnel.
In summary, extra-anatomic vascular bypasses should be used for the aforementioned indications. With separation of vascular repairs from soft tissue defects, all options for wound care are possible—repeated debridement and pack changes, use of a vacuum-assisted device, and early coverage with a myocutaneous flap or split-thickness skin graft.
Footnotes
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Commissioned; internally peer reviewed.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.